Virus and air humidity #01
There are many myths and falsehoods circulating about virus spread, and we believe it is important to stay objective and look at the actual findings of science.
In this first article, we take a look at some of the results achieved by research into the way that air humidity affects the transmission of virus from one person to the next.
What is a virus?Basically, a virus is a package of DNA encased in a protein container. It is debatable whether or not a virus is a living thing – it depends on your definition of “living.” But viruses can only multiply inside a host, and for that reason they have evolved to feature some very clever mechanisms on their outer shell to penetrate immune systems and infect humans and animals. Because they evolve rapidly, every year sees its own outbreak of a flu epidemic – last year’s model has, so to speak, thought up a new way of defeating our defenses. Some years are worse than others, of course. |
Heated rooms = low air humidity
In temperature climates around the world, we heat our homes. This also means that the relative air humidity drops – from a comfortable 50-60 % to less than 20 %. This dry air is uncomfortable for us, it dries out the mucus layers in our respiratory system, and this is believed to be one of the main reasons why flu virus tends to affect us more strongly in the winter months.
But there are many other factors at play, and one is the way virus is spread from an infected host to a non-infected recipient.
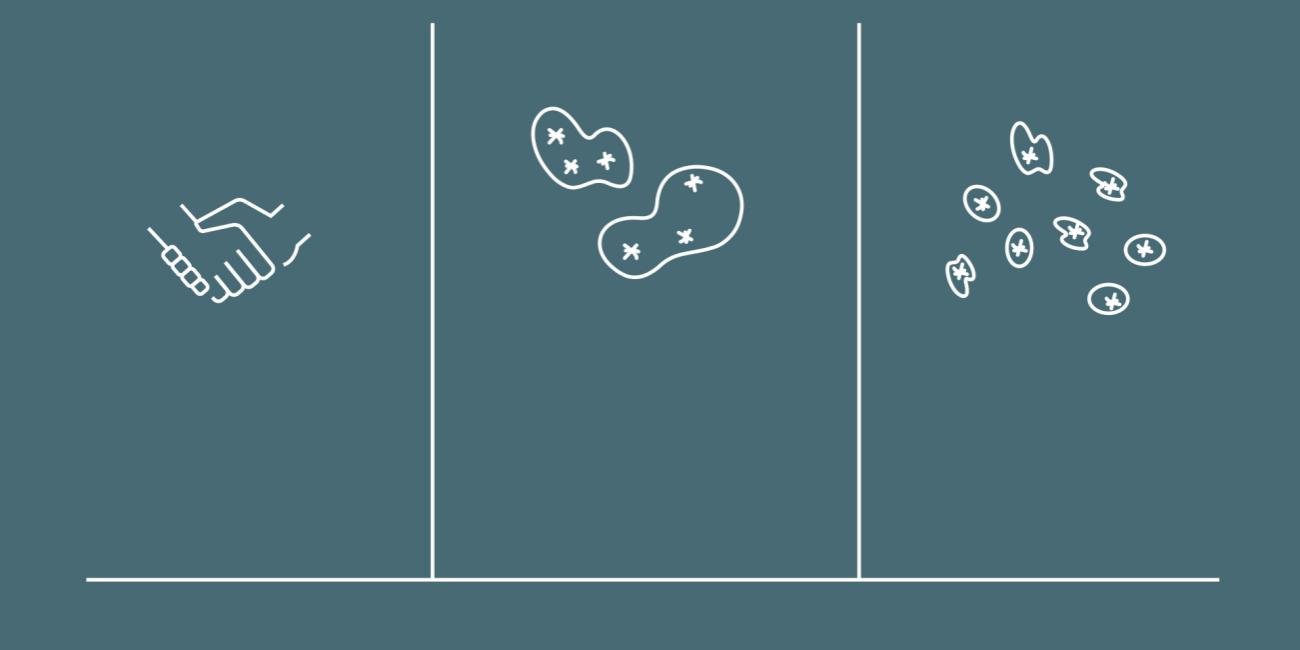
Virus is transmitted in three different ways: By physical contact, in water droplets or in aerosol form.
Three ways a virus can spread
Generally speaking, there are three ways in which a virus can travel from an infected person to a healthy one:
- By touch, e.g. hand-to-hand contact or by someone touching a surface where the virus has been deposited (like a car steering wheel or such).
- Over short distances by being encased in a water droplet with a size from 10 microns up to a few millimeters (a sneeze releases this type of water droplet)
- Over longer distances by being encased in aerosols, i.e. very small water droplets under 5 microns in diameter
The aerosol droplets, in which virus can survive almost indefinitely, are our concern here.
Virus on surfaces is quickly destroyed
Studies show that a virus on a surface can survive for anything up to 72 hours depending on several circumstances, but that in most cases the virus is destroyed after a few hours. This is why general cleanliness during a virus outbreak is a very good idea – wiping off door handles and tabletops with a disinfectant can reduce the risk of contagion dramatically.
But a virus encased in a tiny droplet in aerosol form can stay airborne for days and weeks, and that is the problem.
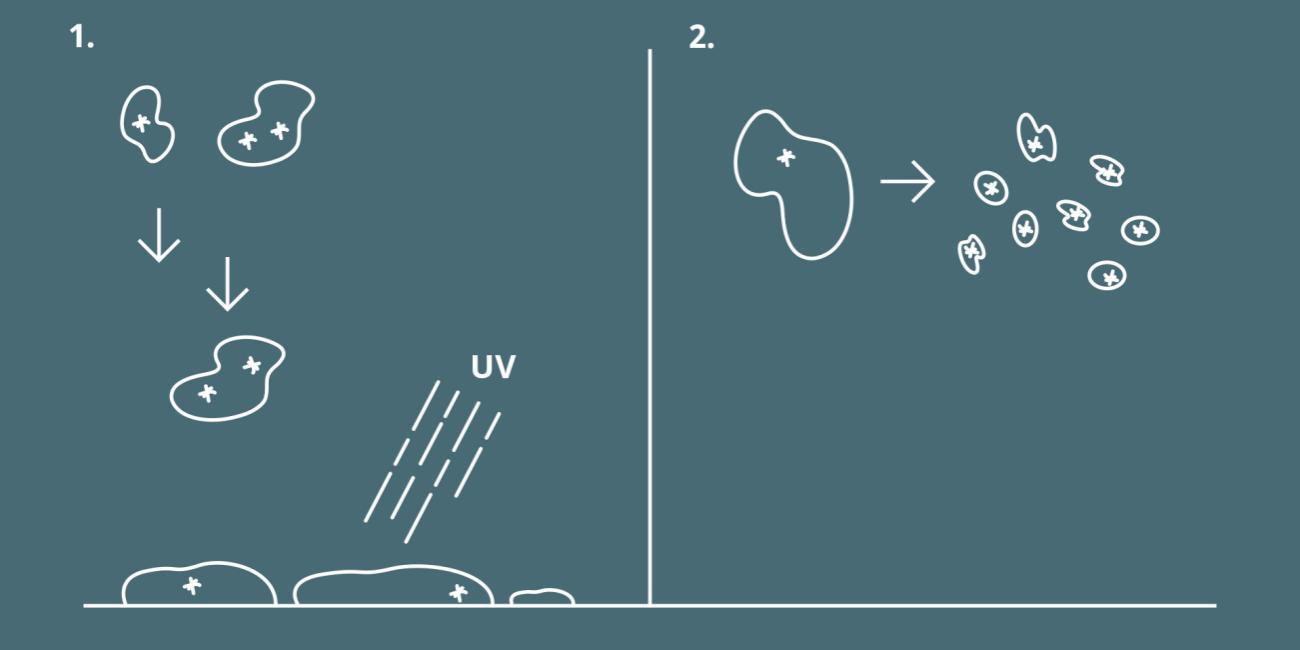
1. Droplets from sneezing or coughing will settle by gravity, provided the relative humidity is correct. Virus will not survive for long on most surfaces.
2. If the relative air humidity is too low, these droplets will evaporate into aerosols with a droplet size of less than 5 micrometers. Such aerosol droplets can carry active virus for hours or even days.
Once a person releases virus via coughing or sneezing, the water droplets containing the virus will begin to fall to the ground until they reach terminal velocity. How long this takes depends on many factors, droplet size not least, but is typically a matter of seconds.
Once the droplet settles on a surface, it eventually dries out, exposing the virus to e.g. UV light, which breaks the virus’s outer shell apart. The virus becomes just a harmless lump of DNA without the means to infect.
Low relative air humidity gives virus better odds for success
In a heated home or office, the relative air humidity is low. Aside from the fact that this is unpleasant to be in, it also means that virus has better odds of staying airborne for longer. In dry conditions, the air is said to be sub-saturated, and this means that larger water droplets tend to evaporate more quickly – turning into an aerosol. Results show that virus encased in aerosol-sized water droplets can stay airborne for half an hour up to a couple of hours, depending on ventilation etc.
Of course, the same UV degradation will take place in an aerosol, rendering the virus harmless after a period of time, but it still a good idea to ensure that the virus emitted from a host settles on a surface as quickly as possible. Otherwise, there is a risk that it will enter the next person via the respiratory tract. Currently, the WHO is investigating this and is likely to implement measures to reduce the risk connected to aerosol-borne virus in e.g. hospitals. 1)
How air humidification can help
So, what is there to be done? We can’t not heat our homes, schools, offices and hospitals. We can’t not sneeze or cough.
But one thing we can do is ensure that indoor air humidity stays at a level where droplets that potentially carry virus will settle quicker by gravity.
In private homes, this can be done by using a small commercial air humidifier, and in larger buildings, this can be done by adding air humidification technology to air ducts and ventilation systems. One the relative air humidity reaches a level of 40 to 60 %, the infectiveness of virus has been documented in many studies to drop dramatically. 2)
This is the reason why air humidification technology is one of the ways we can protect ourselves better against the spread of virus.
Next article is on the way
In our next article, we will show how air humidity levels affect the way we as humans protect ourselves from virus infection. Stay tuned.
More reading
Contact us today and find out more
2) See e.g.: Absolute humidity modulates influenza survival, transmission, and seasonality
Jeffrey Shaman, Melvin Kohn
Proceedings of the National Academy of Sciences Mar 2009, 106 (9) 3243-3248; DOI: 10.1073/pnas.0806852106